Abstract
Background
For patients with symptomatic advanced stage indolent lymphoma, rituximab (R) was traditionally used alongside cyclophosphamide, vincristine, prednisone (CVP), or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Bendamustine plus rituximab (BR) has been found to increase progression-free survival compared to R-CVP/CHOP, albeit with possible increased toxicity. Since 2013, bendamustine has been approved for public reimbursement for treatment of indolent lymphomas in Ontario, Canada, and has become the preferred regimen. We aimed to assess survival and toxicity of patients with indolent lymphoma treated with BR compared with R-CVP/CHOP in Ontario.
Methods
We conducted a retrospective cohort population-based study using administrative healthcare databases from Ontario, Canada. Patients were included if they had a diagnosis of indolent lymphoma and were treated with R-CVP/CHOP from 2005-2012, or with BR from 2013-2018. Patients with mantle cell lymphoma were excluded. Treatment groups were propensity score matched based on lymphoma subtype, age, sex, prior cancer diagnosis, time since diagnosis and rural or urban residence. The primary outcome of the study was overall survival (OS) from time of first treatment to death or end of study period. Secondary outcomes included toxicities during the first nine months of treatment, such as infections and secondary malignancies, associated resource utilization, and outcomes of patients treated with rituximab maintenance therapy. Cox regression models were used to assess factors associated with mortality.
Results
A propensity-matched cohort of 2032 patients treated with BR and 2032 treated with R-CVP/CHOP (1473 patients treated with R-CVP, 559 patients treated with R-CHOP) were included. The median age of patients was 65 years and 48% were female. A majority of patients had a diagnosis of follicular lymphoma (58.9% of patients treated with BR, 59.7% of patients treated with R-CVP/CHOP, p=0.59). Median follow-up time was 41 and 87 months for patients treated with BR and R-CVP/CHOP, respectively.
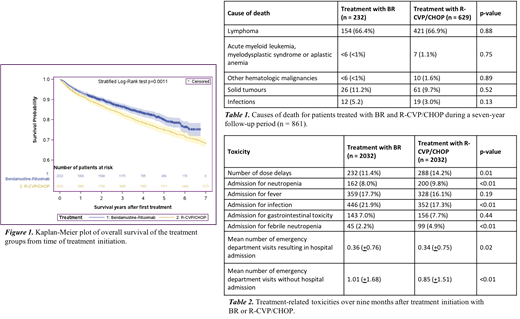
BR was associated with significantly lower mortality compared to R-CVP/CHOP (HR 0.77, 95% CI 0.67-0.89, p<0.01). Five-year OS was 80% and 75% for patients treated with BR and R-CVP/CHOP, respectively (Figure 1). A total of 969 patients died during a follow-up period of seven years - 331 (16.3%) treated with BR and 638 (31.4%) treated with R-CVP/CHOP. Causes of death were available in 861 patients (88.9%), shown in Table 1. Most patients in both groups died from lymphoma (66.9% treated with BR, 66.4% treated with R-CVP/CHOP, p=0.88). A Charlson comorbidity index greater than or equal to 2 was associated with increased mortality (HR 3.04, 95% CI 2.45-3.77).
Treatment toxicities are listed in Table 2.Over a nine-month period after treatment initiation (induction), patients treated with BR had a mean of 0.85 emergency department visits compared to a mean of 1.01 for patients treated with R-CVP/CHOP (p<0.01). Admissions for febrile neutropenia were more common in patients treated with R-CVP/CHOP compared to BR (4.9% vs. 2.2%, p<0.01), whereas infections resulting in hospitalization were more common in patients treated with BR. (21.9% vs. 17.3%, p<0.01).
A majority of patients in both cohorts received maintenance therapy (81% of patients treated with BR, 64.1% of patients treated with R-CVP/CHOP). The use of maintenance therapy with either regimen was associated with less mortality (HR 0.16, 95% CI 0.14-0.19). Among patients who received maintenance therapy, patients previously treated with BR had more hospital admissions for fever (21.5% vs. 18.3%, p=0.03) and infections (37.9% vs. 31.5%, p<0.01) over a three-year period.
Conclusion
In these well-matched cohorts of patients with indolent lymphoma, improved OS was seen with BR compared to R-CVP/CHOP. Patients treated with BR had more infectious complications, although more episodes of febrile neutropenia were seen in patients treated with R-CVP/CHOP. The use of maintenance therapy was associated with less mortality, although patients who received maintenance therapy may have been a more fit and selected population. Higher infectious toxicities were seen for patients who received maintenance rituximab after BR. This study supports BR as the standard of care, and future prospective studies should evaluate the survival and toxicity impact of rituximab maintenance.
Prica: Astra-Zeneca: Honoraria; Kite Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal